43 fda structured product labels
Structured Product Labeling Validation Rules - Food and Drug Administration Guidance for Industry - Indexing Structured Product Labeling (Final) Guidance for Industry: Self-Identification of Generic Drug Facilities, Sites, and Organizations ... 4 Drug Labeling, Listing ... DailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations.Instead, these archives have been split into multiple parts. The remainder archive files consist of bulk ingredient labels, vaccine labels, and some labels for medical …
› industry › fda-data-standards-advisoryStructured Product Labeling Resources | FDA Aug 17, 2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information.
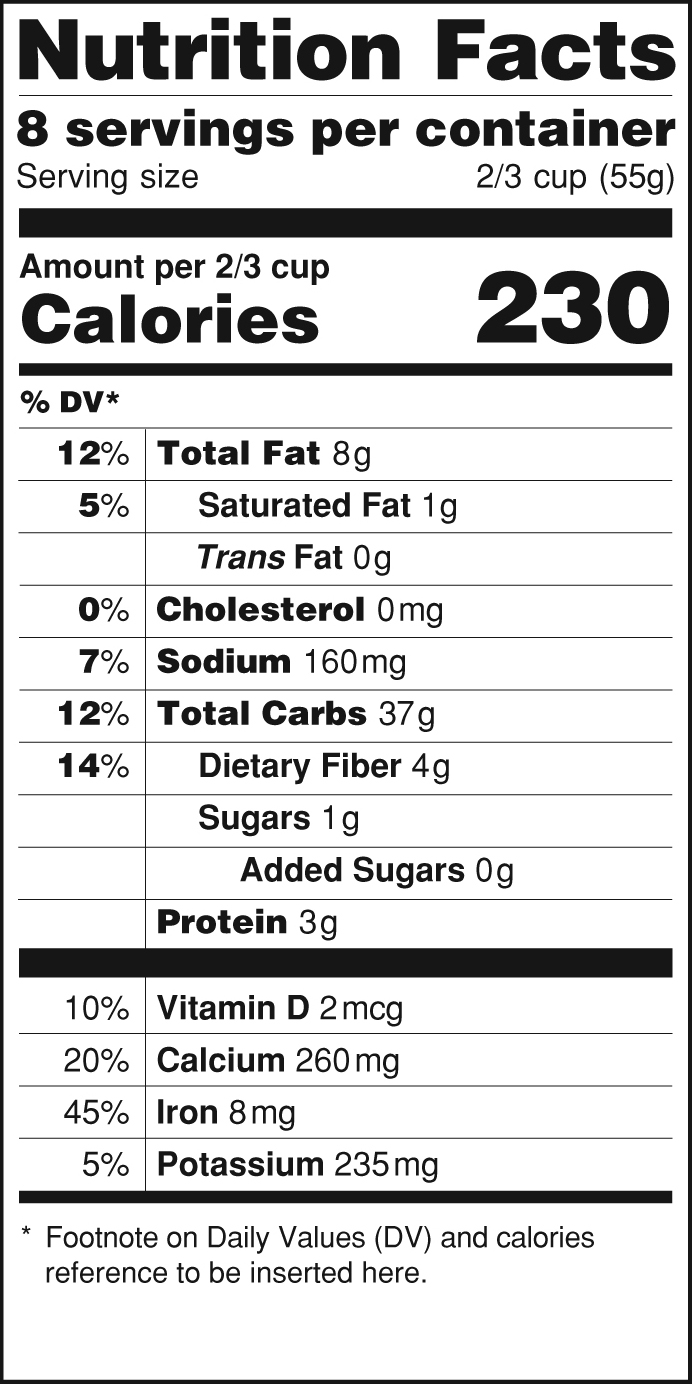
Fda structured product labels
A dataset of 200 structured product labels annotated for adverse drug ... The Structured Product Labels (SPLs), the documents FDA uses to exchange information about drugs and other products, were manually annotated for adverse reactions at the mention level to ... What is Structured Product Labeling (SPL)?, HL7, FDA, Regulatory ... Structured Product Labeling (SPL) is a standard document approved and issued by Health Level Seven (HL7) to exchange information related to product and facility. It is used as a base for Regulatory guidance document in exchange for product labeling content. MTHSPL (FDA Structured Product Labels) - Synopsis Authority The U.S. National Library of Medicine (NLM) produces the Metathesaurus FDA Structured Product Labels (MTHSPL), which is based on the Food and Drug Administration (FDA) Structured Product Labeling (SPL). Information for this source is extracted from the NLM DailyMed Web site. Purpose
Fda structured product labels. Drug Labeling Overview - Food and Drug Administration Drug manufacturers and distributors submit documentation about their products to FDA in the Structured Product Labeling (SPL) format. The openFDA drug product labeling API returns... Exploring Novel Computable Knowledge in Structured Drug Product Labels ... Drug product labeling standards are written into federal law and administered by the FDA. Since 2006, the Code of Federal Regulations has required submissions be sent to the FDA in an electronic format known as Structured Product Labeling (SPL). 17 The SPL format is intended to make labels readable to both computers and humans. FDALabel: Full-Text Search of Drug Product Labeling | FDA The FDALabel Database is a web-based application used to perform customizable searches of over 140,000 human prescription, biological, over-the-counter (OTC), and animal drug labeling... MTHSPL (FDA Structured Product Labels) - Statistics FDA Structured Product Label imprint attribute for shape text: 18077: BLA: Therapeutic Biologic Applications number for the MTHSPL drug: 15324: NDA: New Drug Application number for MTHSPL drug: 11751: DCSA: Controlled Substance Act designation code (e.g. 0,2,3n) 7193: MARKETING_EFFECTIVE_TIME_HIGH:
MTHSPL (FDA Structured Product Labels) - Metadata Unified Medical Language System (UMLS) UMLS Quick Start Guide; FAQs; Customer Support; UMLS Vocabularies MTHSPL (FDA Structured Product Labeling) Source Information Authority The U.S. National Library of Medicine (NLM) produces the Metathesaurus FDA Structured Product Labels (MTHSPL), which is based on the Food and Drug Administration (FDA) Structured Product Labeling (SPL). Information for this source is extracted from the NLM DailyMed Web site. Purpose SPL Xforms | FDA - U.S. Food and Drug Administration To register, please submit the following information via e-mail to spl@fda.hhs.gov: Attendee's first and last name. Name of your organization. E-mail address. Session name and date of training ... FDA SPL - Structured Product & Drug Labeling Composition Process | Reed ... Structured Product Labeling (SPL) is a Health Level Seven (HL7) International standard for regulatory guidance documents as a method for communicating product and facility information. Accepted by the Food and Drug Administration, structured product labeling enhances the cohesiveness and honesty of product information because it requires ...
› industry › structured-product-labelingNSDE | FDA - U.S. Food and Drug Administration Mar 31, 2022 · With the exception of the billing unit data in the NSDE document, this file is generated from SPL documents sent to FDA for inclusion in the FDA Online Label Repository at labels.fda.gov. FDA SPL for SPL R4 - Structured Product Labeling - User Manual Introduction to the Software. FDA SPL is a software that aids pharmaceutical companies and the repackaging/ re-labeling Industry to author SPL files needed to register with US Food and Drug Administration.. The application allows easy document authoring by displaying SPL structured data in a comprehensive tree view. Structured Product Labeling (SPL) | Data Conversion Laboratory - DCL Structured Product Labeling (SPL) is a standard used by the FDA community to facilitate the communication of drug labeling data reliability among various groups such as the FDA, hospitals, prescribing organizations, doctors, and the general public. SPL is an HL7 and ANSI approved standard. Automatic Classification of Structured Product Labels for Pregnancy ... The Code of Federal Regulations (CFR) Title 21 from the Federal Drug Administration (FDA) describes the specific requirements on content and format of labeling for human prescription drug and biological products 4.It includes specific guidelines for a section on specific populations describing the effects on pregnancy and lactation.
› vaccines › programsIIS COVID-19 Vaccine Related Code | CDC The following vaccine NDCs and associated tradenames have been either submitted for FDA authorization (Pre-Authorization) or have been authorized or approved by the FDA under EUA or BLA License and may be included in FDA NDC files and Structured Product Labels (SPL).
Medical Condition | FDA - U.S. Food and Drug Administration It allows a consistent way to index, store, retrieve, and aggregate clinical data across specialties and sites of care. SNOMED CT is used to represent Medical Condition in Structured Product...
› fintech › cfpb-funding-fintechU.S. appeals court says CFPB funding is unconstitutional ... Oct 20, 2022 · Twitter’s product leaders are already trying to think through it with the goal of potentially relaunching by the end of the year. An early idea is to turn the camera inside of Twitter’s app into an easy way to record and post Vines, according to a report from Platformer. A reported use case could be users filming reaction videos to tweets ...
DailyMed 15.09.2021 · The National Library of Medicine (NLM)’s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i.e., "in use" labeling). DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical …
Assessing the Impact of HL7/FDA Structured Product Label (SPL) Content ... To understand the impact of SPL labels for current drug knowledge management and CPOE system implementation, this paper investigates (1) if SPL labels are sufficient as an exclusive source for drug information for e-prescribing systems today; (2) if SPL labels can be used directly in conjunction with other knowledge sources.
› publication › ppic-statewide-surveyPPIC Statewide Survey: Californians and Their Government Oct 27, 2022 · Key Findings. California voters have now received their mail ballots, and the November 8 general election has entered its final stage. Amid rising prices and economic uncertainty—as well as deep partisan divisions over social and political issues—Californians are processing a great deal of information to help them choose state constitutional officers and state legislators and to make ...
Structured Product Labeling Improves Detection of Drug-Intolerance Issues Introduction and Objective. The HL7 Structured Product Labeling (SPL) standard 1 implemented by the FDA uses the HL7 Reference Information Model (RIM) 2 to represent the chemical and physical nature of medical products and their safe and effective use. While not all of this content is available today, we enrich the 3704 available SPLs with knowledge from the SPL terminology sources, including ...
Structured Product Labeling - Food and Drug Administration Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. The...
SPL Standard Training | FDA A series of Structured Product Labeling (SPL) training opportunities are being offered to individuals responsible for the preparation and submission of information to be provided in SPL format to ...
labels.fda.govFDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.)
Introduction to FDA Structured Product Labeling - SPL R4 SPL. The Structured Product Labeling (SPL) specification is a document markup standard that specifies the structure and semantics for the regulatory requirements and content of the authorized published information that accompanies any medicine licensed by a national or international medicines licensing authority. UCUM.
FDA Label Search-Proprietary Name - Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 Ph. 1-888-INFO-FDA (1-888-463-6332) Contact FDA For Government For Press Combination Products Advisory Committees Science & Research...
DailyMed - FDA Resources: SPL, Other Prescription Drug Labeling ... Structured Product Labeling (SPL) is the standard format for electronic submission of the content of labeling. For SPL resources (including industry data standards for SPL), see FDA's SPL Resources page and the "Structured Product Labeling Resources" heading on FDA's Prescription Drug Labeling Resources page.
Indexing Structured Product Labeling | FDA Center for Drug Evaluation and Research Center for Biologics Evaluation and Research This guidance explains that FDA's Center for Drug Evaluation and Research (CDER) and Center for Biologics...
Structured Product Labeling - Wikipedia Structured Product Labeling ( SPL) is a Health Level Seven International (HL7) standard which defines the content of human prescription drug labeling in an XML format. [1] The "drug labeling" includes all published material accompanying a drug, such as the Prescribing Information which contains a great deal of detailed information about the drug.
FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor verified by FDA. The drug labeling …
› ohrp › regulations-and-policy45 CFR 46 | HHS.gov The HHS regulations for the protection of human subjects in research at 45CFR 46 include five subparts. Subpart A, also known as the Common Rule, provides a robust set of protections for research subjects; subparts B, C, and D provide additional protections for certain populations in research; and subpart E provides requirements for IRB registration.
MTHSPL (FDA Structured Product Labels) - Synopsis Authority The U.S. National Library of Medicine (NLM) produces the Metathesaurus FDA Structured Product Labels (MTHSPL), which is based on the Food and Drug Administration (FDA) Structured Product Labeling (SPL). Information for this source is extracted from the NLM DailyMed Web site. Purpose
What is Structured Product Labeling (SPL)?, HL7, FDA, Regulatory ... Structured Product Labeling (SPL) is a standard document approved and issued by Health Level Seven (HL7) to exchange information related to product and facility. It is used as a base for Regulatory guidance document in exchange for product labeling content.
A dataset of 200 structured product labels annotated for adverse drug ... The Structured Product Labels (SPLs), the documents FDA uses to exchange information about drugs and other products, were manually annotated for adverse reactions at the mention level to ...

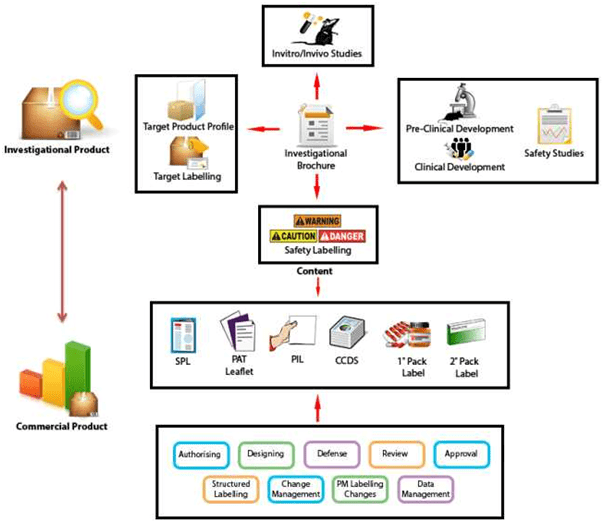




![Food Labeling 101 - FDA Regulations Guide [2022] | Artwork Flow](https://global-uploads.webflow.com/5f59aa263c234bb74025de57/5fa4f81b6e365340866e0eeb_Inner-Images-4.jpg)





.png.aspx)
.jpg)












Post a Comment for "43 fda structured product labels"